Clinical Research Data Request Form
Create New Request : Please click on Create New Request to create and submit Research proposal requesting for Clinical Trial data
Update Existing Request : Please click on Update Existing Request to update an existing Request. Please enter the system generated Request ID and Requestor email address who created/submitted the request. In an event of document(s) update e.g., Research Proposal, CV of researcher(s), etc please upload the full document(s).
Request Contact : For questions regarding the request form or approval status or request cancellation, please contact dataaccess@msd.com
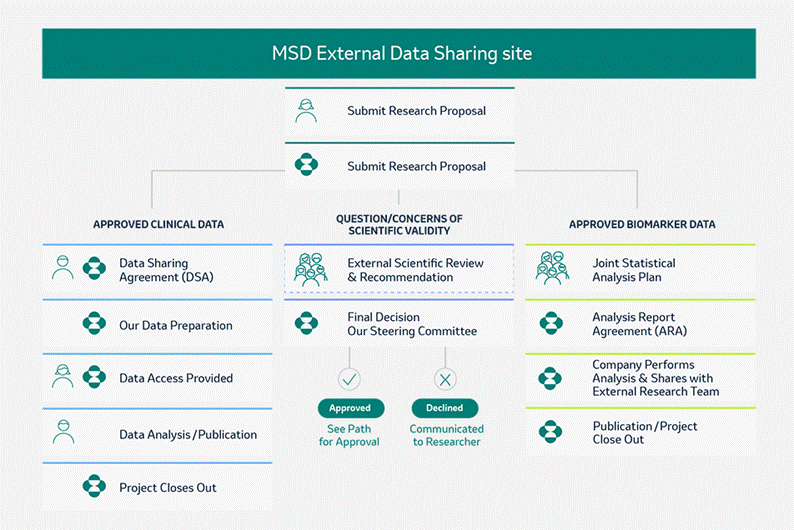
Data Access - External Data Sharing
OBJECTIVES
Merck provides access for qualified external(i.e non-MSD) researchers to patient-level data and CSRs, including biomarker data, for clinical trial performed by Merck for which results are posted on the clinicaltrials.gov registry (dating back to September 2007) for products or indications that have been approved by regulators in the US and EU. In general, data are made available for request approximately 18 months after clinical trial completion.
*Data from Phase I trials in healthy volunteers and consumer health care studies are out of scope.
Genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and Company subject matter experts.
The MSD Data Sharing policy can be accessed by clicking the following link: ProcedureAccessClinicalTrialData.pdf
For technical issues, please contact crdsrs-ams@msd.com